I’m doing the damn thing and finally embarking on a PhD journey! Ever since I transitioned from working in industry to working in academia a little over 3 years ago now, I’ve been slowly cracking away at a master’s degree in chemistry and I finished this December. So now I’m working on a PhD for the foreseeable future.
Won’t it be
a lot of extra work? Yes, it will.
Don’t I
already have a full-time job? Yes, I do.
Why am I
doing this?!
Because:
a.) I want the freedom to do whatever I want for my entire career, and I believe having a PhD will help to enable that kind of freedom.
b.) Learning is a spiritual journey for me. This is how I make the world a better place. I believe learning raises the vibration of the oneness, ie: it makes God happy. It feels like this is my mission on this planet: to collect as much knowledge and experience as possible and dissolve it back into the source. I think the universe loves to be examined and explored. She’s beautiful and sexy and she loves to be checked out. She wants us to find out every little thing about her. The universe loves to be loved and appreciated. Haven’t you ever noticed that when you intentionally shift your mindset to one of gratitude, your life starts going better? When you love and appreciate the universe, she will love and appreciate you right back. Every human, animal, mineral, plant, piece of technology…we’re all just little fractions of the God holograph lovingly gazing at each other, trying to take it all in. Learning is a spiritual act of love for me and it’s what I’m here on this planet to do.
c.) The research area of the lab I joined is renewable energy, which feels like a noble cause. What could be a more important pursuit in the face of impending planetary doom?
d.) The principal investigator in this group is ambitious as hell. He’s a big ideas kind of guy and he gets shit done. I think watching how he works will be inspirational.
So here I am to write about what it is that this lab does because explaining things to “other people” in writing is the best way that I learn (and when I refer to “other people”, I mean mostly myself but also the bots that leave comments on my blog posts with sketchy links that probably gave me a million viruses when I clicked on them.) And, to be honest…so far, I don’t actually know much about what they do in that lab other than they all seem really smart, and it has something to do with renewable energy. But there are all kinds of topics that fall under the category of renewable energy. What does this group do in particular? I don’t really know, let’s take a look at the content they have online and do a little copy/paste, shall we?
“The
Boettcher electrochemistry and solar materials
laboratory is focused on designing, synthesizing, and understanding materials
for applications in solar energy conversion and electrochemical energy
storage/conversion. Specific interests include the synthesis and study of
heterogeneous electrocatalysts for water oxidation with defined molecular and
nanoscale structures, the use of computer simulation and direct electrical
measurements to understand semiconductor-electrocatalyst interfaces, and the
development of high-performance III-V semiconductor solar conversion
architectures using scalable and inexpensive deposition processes. Recent new
projects include the development of alkaline membrane electrolyzers for
low-cost scalable hydrogen production as well as fundamental aspects of bipolar
membranes and electrolyzers.”
Lots of big scary
words in there. I need to break this paragraph down word-by-word to absorb it.
Let’s start with heterogeneous electrocatalyst.
A catalyst
is a chemical that increases the rate of reaction without itself undergoing any
significant physical change.
An electrocatalyst
is a specific type of catalyst that functions at an electrode surface. So, on
the surface of some conductor that can be hooked up to a power supply and charged up.
A heterogeneous
catalyst means the phase of the reacting chemical at the beginning of the
process is different from the phase at the end.
Changing
phase…does that mean we are going from a solid to a liquid? Or a liquid to a
gas? Yes, that’s exactly what it means! In fact, here, we are talking about
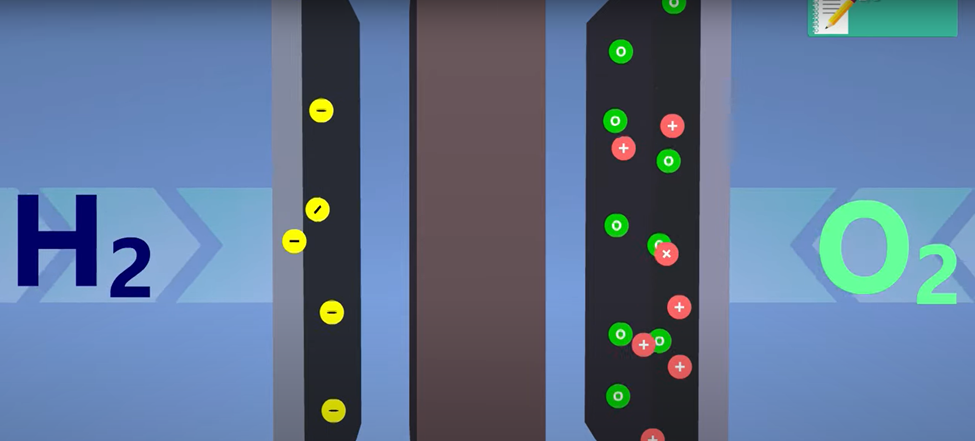
water oxidation, which is the process of splitting water into oxygen and hydrogen.
This is a liquid turning into a gas.
Note, hydrogen
is a proton. I’m going to use those two words interchangeably throughout this
piece. Don’t get confused!
So, to sum
up the first half of that big, wordy paragraph: this lab is trying to create
and test materials that use electrical and chemical reactions to turn water
into hydrogen gas and oxygen gas. This process is called water electrolysis.
Why would we
want to turn water into hydrogen gas?
Because,
when used in a fuel cell, hydrogen gas generates electrical power and emits only drinkable
water and warm air. It’s just about as efficient as gasoline and has near zero greenhouse
emissions.
How does a
fuel cell work?
There are a bunch of different technologies for fuel cells, I'm going to explain how PEM fuel cells work.
This video gives a pretty good introduction. I grabbed some screenshots from that YouTube to explain what’s going on.
A fuel cell
is essentially a sandwich with two metal plates (an anode and cathode) as the
bread, and this thing called a Proton Exchange Membrane (PEM) in between.
The PEM is a
material made from a special type of polymer designed to let through protons and
block electrons. I am not clear on how it does that, exactly! It looks like
I’ll need to teach myself some serious chemistry to begin understanding what’s
going on here.
Let’s start with
understanding what a polymer is. Well, it’s just a material that’s made from
large molecules consisting of a bunch of repeating subunits. I remember my
biology 101 professor holding up a stack of Legos as an analogy to polymers;
Little repetitive building blocks that link together to make a larger
structure.
DNA is a polymer! It’s just a long, twisted
ladder with the same 4 components repeating themselves in different
combinations over and over. There are also ton of synthetic polymers:
polyesther, polystyrene, nylon, Teflon…the list goes on and on.
Proton
Exchange Membranes are made of a special type of polymer called an ionomer, which
means that some of the subunits sprinkled throughout the matrix have an
electric charge to them. Often, a product called Nafion is used.
The chemical
structure of Nafion looks like this:
So…what’s going on here with this big jumble of sticks and letters? I’ve never taken organic chemistry, so this is a lot for me… but what I’m gathering is that when you take this big ugly ionomer structure and soak it in water, the part that I circled in pink (the sulfonic acid group) loses protons and those protons start hopping around from one acid site to another. If you apply an electrical bias, you can get all the protons to move in one direction. This is an electrical current!
Pressurized hydrogen from the fuel is forced through the anode while pressurized oxygen from the atmosphere is forced through the cathode.
An acid is
used as a catalyst to split the H2 molecules into protons and electrons.
I had to go into a Wikipedia worm hole to remind/teach myself what it means to be acidic. Remember how the pH scale works? I didn’t. Here’s what I found out: pH is an acronym! It stands for potential of hydrogen. Acids have high concentrations of free hydrogen ions floating around, which means they are hungry for electrons. An acid will slurp up the electrons from the hydrogen, leaving only positively charged protons, which can move across the Nafion membrane.
Ok, so now the protons have made their way across the membrane to hang out with the oxygen molecules on the right side of the fuel cell and they leave their electrons behind on the left side.
That means the right side has a positive charge and the left side has a negative charge. This is a battery! This is exactly what a battery does, it’s just a separation of charge that creates a potential difference. If we connect a circuit between the two charged plates, we can force electrons through the circuit. This will power whatever we’ve got going on in the circuit.
As an aside, I’d like it to be known that this technology is yet another one of the many gifts given to us by the space industry! Tons of resources were devoted to the study of these Proton Exchange Membranes (PEMs) during NASA’s Gemini project in the 1960’s.
I get on my soap box about this all the time. Space exploration truly is the gift that keeps on giving. Space travel presents us with novel problems that require creative solutions. This forces the development of innovative new technology that we end up using for all kinds of other applications. The list of technology that came from the space industry goes on and on. I don’t know why so many people get all up in arms about how we shouldn’t be devoting resources to space exploration, but those people annoy me.
Anyway…space rant over. Back to fuel cells!
Why are fuel
cell vehicles way better than battery powered vehicles?
It takes
hours to fully charge a battery powered vehicle and even with a full charge you
can’t go that far. Most electric cars can barely get half as far as a car with
a full tank of gas. And then when the battery runs out, you have to wait around
for a long time to recharge it. Hydrogen cars can go just as far as
gasoline-powered cars, and you can refill the tank with hydrogen in the same amount of time
that it takes to refill a car with gasoline. There are already hydrogen powered cars cruising the streets in California.
But we
aren’t just talking about cars for fuel cell applications; Trains, planes,
spacecraft, power for big industrial settings…all of this could be powered by
hydrogen fuel cells to create a completely green economy.
Sounds
great, right? A battery that works better and takes less time than the current Lithium-ion
batteries and is also completely carbon neutral?
Wrong! Hydrogen
fuel only counts as renewable energy if the process of making the hydrogen is renewable.
Currently,
hydrogen is primarily made through steam-methane reforming. This is a process
where high-temperature and high-pressure steam is combined with natural gas to
create hydrogen gas. Natural gas is not all that green…
So, this lab’s goal is to develop green technology for creating hydrogen fuel using a process called water electrolysis. We want to mimic the process of photosynthesis, using solar energy to split water into oxygen and hydrogen and store the hydrogen molecules as fuel to power fuel cells.
As far as I
can tell, the biggest rock star in the group is this woman named Grace. She takes responsibility for getting stuff done and moving the group forward. She’s graduating in spring which
is going to be a big loss to the group, but an enormous gain to the organization
she goes on to work for. She spent a couple hours with me explaining how water electrolysis works. Here's what I learned:
Currently
there are three different technologies out there for water electrolysis and
they each have some big problems.
We’ve got:
1.) Liquid alkaline
2.) Proton Exchange Membrane (PEM’s…yay we already learned about those above!)
3.) Anion Exchange Membranes (AEM)
So, how do each of these technologies work? Or I suppose I should say…how don’t they work?
Let’s start with liquid alkaline. I learned a lot from this cute Spanish lady. I included a screen shot of her talk below with a cartoon of the liquid alkaline electrolyzer. It's helpful to refer to the cartoon while I explain how it works.
In this type
of device, you have two metal plates separated by a porous foil that won’t let
electrons through; only OH- ions (these are called hydroxyls). I’m
not going to do a deep dive on how that foil transports ions, but I suspect
it’s some kind of polymer that forces the OH- to hop from molecule
to molecule, kind of like I described above with the acid group in the PEM. It
also isn’t supposed to let gas through; only liquid. I guess this is achieved
mechanically with design of the porous foil. The whole thing is submerged in a
highly concentrated solution of potassium hydroxide, which is strongly alkaline.
Alkaline is
on the opposite end of the pH scale from acids, instead of a bunch of free
hydrogen floating around, it has free hydroxyl ions. Instead of wanting to
steal electrons, it wants to steal protons.
So anyway, this device is submerged in a liquid that has a strong concentration of hydroxyl ions floating around, and its functionality leans heavily on those hydroxyls flowing through the membrane to react with stuff on the other side. In this case, we have water flowing in on the right side of the cell. Two water molecules react with two electrons to form hydrogen gas and two hydroxyls.
Why does the
water react with two electrons and break down into its components? Does water
just do this on its own? No, thankfully! Water doesn’t just spontaneously fall
apart else we’d be in trouble considering how much water our bodies use to keep
us alive. We need to force this reaction to take place by introducing
electricity into our system. When you apply a huge voltage to water and add a
little bit of electrolyte (the potassium hydroxide), you can force a current through the water which
pulls electrons away from the solution at the cathode while forcing electrons
into the solution at the anode.
Great, so
now on the right side we have water flowing in and reacting with electrons from
the electrode to create hydrogen gas and hydroxyls. Hydrogen gas is our product! It
bubbles out and gets stored. The
hydroxyls are a liquid and flow over to the left side through the membrane
where they become water, oxygen, and electrons. The oxygen gas bubbles out,
this is a secondary product! The electrons make their way over to the positive
side via a circuit and this supplies the electrons to continue the process on
the right side.
Liquid
alkaline electrolyzers are great because they are low cost and reliable. They
are already used all over the place in big industrial settings for hydrogen
production and ammonia synthesis for fertilizer. Unlike the other two
technologies we will highlight below, they can be made of materials that are
abundant on earth and cheap, like nickel!
They suck
because they suffer from this problem called “shunt current”. This is when
current starts going through the tubing instead of the electrode. This becomes
more of a problem as you increase the current: more of it goes through the
wrong pathway. This is a problem is exacerbated because liquid alkaline electrolyzers have to be constructed as a huge stack containing multiple electrodes
in series.
Another huge problem that can occur when you vary the current is that instead of bubbling out, the gas tends to cross over the microporous diaphragm and hydrogen and oxygen gas will mix. You know what happens when you mix hydrogen and oxygen?
 |
| This is a picture of an industrial hydrogen plant in China that went boom. |
So, because
of shunt currents and gas cross-over, you must have a steady current and
therefore a non-variable power supply.
Well, you
can’t really ask the sun to shine or the wind to blow in a non-variable way,
therefore this technology can’t be paired with renewable energy. So, if we
stick with liquid alkaline electrolyzers, water electrolysis is never going to
be renewable.
X Liquid alkaline electrolyzers are out! They can't be paired with renewables!
What’s next?
Next up, we
have Proton Exchange Membranes (PEM’s), which I described in depth above when I
was talking about fuel cells. When we were talking about it above, it was
hydrogen in, electron current and water out. But now it’s electron current and
water in, hydrogen out.
Look at this
cute cartoon I found on Wikipedia! Staring at this for a while helps a lot!
The fact that the thing in the middle is a membrane rather than a porous thing means we don’t have the gas cross-over problem that acid alkaline electrolyzers have. AND, we don’t have to have a bunch of electrodes in a stack so we don’t have the shunt current problem either. Great, so this technology is both safer and able to be paired with renewable energy!
So, what’s
the downside with PEM’s? Well, instead of using an alkaline as a catalyst, we
are using an acid. This is a problem because acid will chew away most metals.
The only metals that are stable in an acidic environment are precious metals
like iridium. Since iridium is one of the scarcest metals on earth, this is the
bottle neck in PEM electrolyzer production.
X PEM’s won’t
work either. They require metals that are too scarce and expensive!
Finally, onto Anion Exchange Membranes. Instead of exchanging protons through the membrane, we exchange hydroxyls...just like with the liquid alkaline electrolyzers! Hydroxyls are ions with a negative charge, the word for that is anions, this is where we get the name Anion Exchange Membrane. These devices get rid of the problems created by PEM’s because they can be used in an alkaline environment rather than an acidic environment. That means we can construct these with earth-abundant metals.
The biggest
problem for AEM’s is poor durability.
Many
different polymers have been tried out as the anion-conducting membrane and
they are all trash! AEM’s just fall apart faster than PEM’s and no one is totally clear
on why that is. It sounds like my first project in this lab is supposed to be trying to understand how/why these things break.
In the AEM
community, it had been thought that the problem with these devices is that the
membrane is limiting. However, it’s interesting that the rate of degradation is
the same no matter what material the membrane is made from. To get a grip on
what’s going on, researchers started incorporating a reference electrode in the
middle of the stack. From this electrical information, they learned that it’s
not the membrane that’s degrading, it’s the anode!
So, what I'll do is use my Focused Ion Beam to cut a bunch of cross-sections of used devices and unused devices. We’ll compare the two and see if there is any obvious physical degradation on the microscale. Hopefully, understanding how these devices are breaking will help us to make them stronger.
And that’s it! That’s what I’ll be working on for the foreseeable future (on the side of my regular full time job, that is)
So far, I've sliced up a healthy AEM and put all the slice images together into a movie. Here's what it looked like. Next up, I'll work on a used AEM to look for differences and make improvements on my observation methods.
If you read this far, I am impressed and grateful for your mental fortitude! That's enough nerding out for now. See ya next time!